Die Reform des EU-Arzneimittelsektors ist ein Meilenstein der Europäischen Gesundheitsunion und ein entscheidender Schritt hin zu einem gesünderen, krisenfesteren und gerechteren Europa. Sie bietet Gelegenheit, diesen zentralen Sektor agiler und flexibler zu gestalten und dem Bedarf des 21. Jahrhunderts zu genügen. Es ist die größte Reform seit über 20 Jahren.
Der Zugang zu Arzneimitteln variiert innerhalb der EU. Manche müssen im Schnitt vier Monate auf ein bestimmtes Medikament in ihrer Apotheke warten, während andere auf dasselbe Arzneimittel über zwei Jahre warten müssen. Darüber hinaus wächst die Sorge vor möglichen Engpässen bei Antibiotika oder Schmerzmitteln.
Die EU-Kommission will den Pharma-Sektor daher mit einem patientenzentrierten Konzept modernisieren, in dem auch eine innovative und wettbewerbsfähige Industrie ihren festen Platz hat. Die hohen EU-Standards für die Zulassung verträglicher, wirksamer und hochwertiger Arzneimittel bleiben hierbei gewahrt.
Was bringt die Reform des Arzneimittelsektors?
- BinnenmarktSchaffung eines Arzneimittel-Binnenmarktes
- Regelungsrahmenweniger Bürokratie, damit Arzneimittel schneller bei den Patient*innen sind
- Medikamente für allebesserer Zugang zu erschwinglichen Arzneimitteln
- ArzneimittelversorgungBeseitigung von Engpässen und Gewährleistung der Versorgungssicherheit
- InnovationFörderung von Innovation und Wettbewerbsfähigkeit
- Umweltfreundlichkeitökologisch nachhaltigere Arzneimittel
- Rettung von MenschenlebenBekämpfung der Antibiotikaresistenz
- Transparenzkonsequentere Aufklärung über öffentliche Gelder in der Pharma-Entwicklung
Alles dreht sich um die Patient*innen

Europaweit sollen alle Patient*innen Zugang zu denselben Arzneimitteln erhalten. Sie sollen die Medikamente erhalten, die sie brauchen — und zwar in allen Mitgliedstaaten. Mit diesem Legislativvorschlag soll allen Menschen ein besserer Zugang zu wirksamen und erschwinglichen Arzneimitteln ermöglicht werden.
Konkret müssen Patient*innen EU-weit rasch und gleichberechtigt auf verträgliche, wirksame und erschwingliche Medikamente zugreifen können. Hierzu werden Anreize für Unternehmen geschaffen, ihre Arzneimittel EU-weit anzubieten. Die EU-Rechtsvorschriften über Medikamente für Kinder und gegen seltene Krankheiten sollen überarbeitet werden.
Ein patientenzentriertes Konzept bedeutet auch, die Versorgungssicherheit zu erhöhen und dafür zu sorgen, dass EU-weit, unabhängig vom jeweiligen Wohnort, stets Arzneimittel verfügbar sind.
Was bringt ein patientenzentrierter Ansatz?
- Leichteren Zugang zu wirksamen und erschwinglichen Medikamenten
- Weniger Engpässe und größere Verfügbarkeit von Arzneimitteln
- Biosimilars gelangen zur Senkung der Arzneimittelpreise früher auf den Markt
- Mehr Medikamente für Kinder und gegen seltene Krankheiten
- Stärkere Patient*innen-Vertretung bei der Zulassung von Arzneimitteln
- Leichteren Zugang zu Produktinformationen
- Ökologisch nachhaltigere Medikamente
Verringerung der Gefahr kritischer Engpässe im Winter und darüber hinaus
Im Oktober 2023 haben wir weitere Maßnahmen ergriffen, um kritische Engpässe bei Arzneimitteln in der EU zu verhindern und zu verringern. Im vergangenen Winter fehlten beispielsweise bestimmte Antibiotika. Damit sich solche Situationen nicht wiederholen, müssen Lieferprobleme ausgeräumt und Lieferketten langfristig robuster werden. Dazu ist nach wie vor ein koordiniertes Vorgehen der EU erforderlich.
Zur besseren Vorbereitung auf den Winter 2023/24 haben die Europäische Behörde für die Krisenvorsorge und -reaktion bei gesundheitlichen Notlagen und die Europäische Arzneimittel-Agentur wichtige Antibiotika ermittelt, um sich im Vorfeld von diesem und künftiger Winter auf die Gefahr kritischer Engpässe einzustellen.
Die Maßnahmen zur Bewältigung von Arzneimittelengpässen in der EU stützen sich auf drei Säulen:
- sofortige und kurzfristige Maßnahmen zur Abmilderung kritischer Engpässe
- strukturelle Maßnahmen zur Förderung der langfristigen Versorgungssicherheit
- Internationale Partnerschaften mit Blick auf die Versorgung
Als Sofortmaßnahme hat die Kommission am 24. Oktober 2023 einen freiwilligen europäischen Solidaritätsmechanismus für Arzneimittel ins Leben gerufen, über den einzelne Mitgliedstaaten ihren Bedarf an einem bestimmten Arzneimittel melden und die übrigen Mitgliedstaaten mitteilen können, mit welchen verfügbaren Arzneimitteln sie aushelfen können.
Weitere Maßnahmen:
- Aufstellung einer EU-Liste kritischer Arzneimittel (bis Ende 2023), um zu analysieren, welche zusätzlichen Maßnahmen in der Lieferkette ausgewählter Arzneimittel erforderlich sind
- Einführung spezifischer gemeinsamer Maßnahmen in 2024, die den Mitgliedstaaten erlauben, Ausnahmeregelungen zu treffen, damit Arzneimittel die Patient*innen besser erreichen
- Organisation des Austauschs bewährter Verfahren zwischen den Mitgliedstaaten in Bezug auf Preisfestsetzung und Erstattung
- Herausgabe von EU-Leitlinien für die Arzneimittelbeschaffung bis Anfang 2024 zur Stärkung der Versorgungssicherheit
- Einführung der gemeinsamen Beschaffung von Antibiotika und Mitteln gegen Atemwegsviren für den Winter 2024/25
Förderung der langfristigen Versorgungssicherheit
Um die Herstellung weiterer kritischer Arzneimittel zu fördern, errichtete die Kommission im Januar 2024 eine Allianz für kritische Arzneimittel. Die Allianz ermöglicht es den nationalen Behörden, der Industrie, den Vertretern der Zivilgesellschaft, der Kommission und den EU-Agenturen, ihre Maßnahmen gegen fortbestehende Arzneimittelengpässe auf EU-Ebene zu koordinieren und Schwachstellen in der Lieferkette zu beheben. Der Schwerpunkt liegt auf den Arzneimitteln mit der größten Gefahr von Lieferengpässen und deren Lieferengpässe die gravierendsten Folgen für Gesundheitssysteme und Patient*innen haben. Mit dieser Maßnahme könnte ein künftiger „Rechtsakt für kritische Arzneimittel“ angebahnt werden. Die ersten Empfehlungen der Allianz zur Verbesserung der Versorgung mit kritischen Arzneimitteln werden für Herbst 2024 erwartet.
Im ersten Halbjahr 2024 werden die Kommission und die Mitgliedstaaten auch ein gemeinsames strategisches Konzept für die Bevorratung von Arzneimitteln entwickeln, um Engpässen vorzubeugen.
Über die EU-Grenzen hinaus werden wir ein Netz von internationalen Partnern knüpfen, um die Belastbarkeit der Lieferkette zu stärken und strategische Partnerschaften mit Drittstaaten für die Herstellung kritischer Arzneimittel aufzubauen, die sowohl die Nachfrage vor Ort als auch den Bedarf auf EU- und globaler Ebene im Blick haben.
Innovationsförderung für eine wettbewerbsfähige Pharma-Industrie

Die EU strebt ein europaweit attraktives und innovationsfreundliches Umfeld für Forschung, Entwicklung und Herstellung von Arzneimitteln an. Hierzu will sie auf der Grundlage stabiler, kohärenter und zeitgemäßer Regeln, die mehr Wettbewerbsfähigkeit und gleichzeitig weniger Bürokratie und Kosten bewirken, Innovationen von Weltrang fördern.
Innovative Arzneimittel sollen schneller zugelassen werden, indem einfachere Vorschriften und Verfahren eingeführt und die Patient*innen stärker ins Arzneimittel-Bewertungsverfahren einbezogen werden, ohne dass das auf Kosten der Sicherheit geht.
Bei Forschung und Entwicklung in Bereichen des ungedeckten medizinischen Bedarfs soll Innovation im Sinne der Patient*innen durch weltweit wettbewerbsfähige Anreize belohnt werden.
Hierzu schlägt die Kommission eine Richtlinie vor, die alle Vorschriften zur Zulassung, Überwachung und Kennzeichnung, zum rechtlichen Schutz, zum Inverkehrbringen und zu anderen Verfahren für alle auf EU- und nationaler Ebene zugelassenen Arzneimittel enthält und die Zulassungsvorschriften EU-weit vereinheitlicht.
Wie wird ein wettbewerbsfähiger Pharma-Sektor Wirklichkeit?
- Förderung von Spitzen-Innovationen
- Weniger Bürokratie und zügigere Zulassung innovativer Arzneimittel
- Anreize für Forschung und Entwicklung
- Bekämpfung der Antibiotikaresistenz
Bekämpfung der Antibiotikaresistenz
Antimikrobielle Mittel sind Arzneimittel zur Vorbeugung und Behandlung von Infektionen bei Mensch, Tier und Pflanzen. Antimikrobielle Resistenzen (AMR) treten auf, wenn Mikroben nicht mehr auf Medikamente reagieren, die sie abtöten sollen. Sie werden verursacht durch den übermäßigen Einsatz oder Missbrauch antimikrobieller Wirkstoffe wie Antibiotika.
Eine lautlose Pandemie
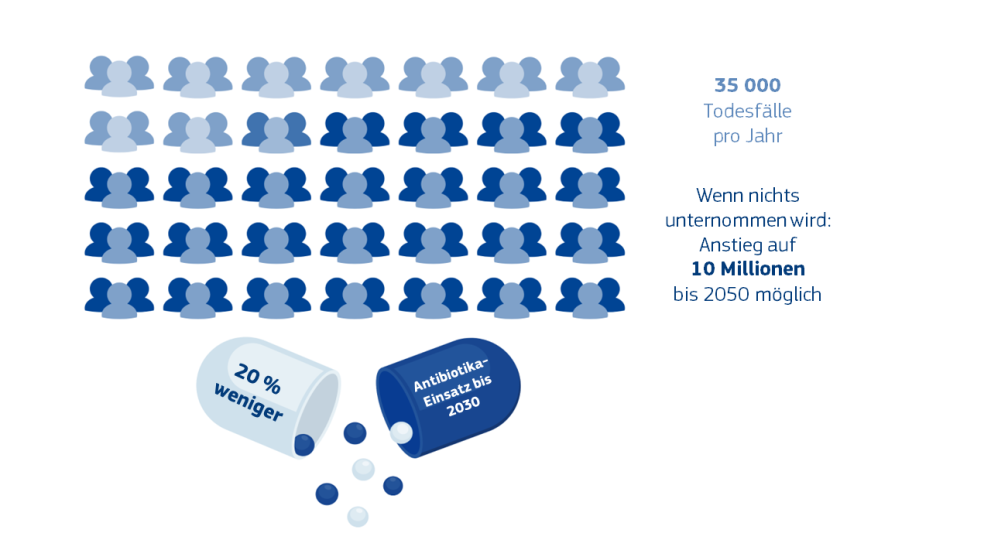
Bedenkt man, dass die Zahl der jährlichen Todesfälle aufgrund von AMR bis 2050 weltweit auf bis zu 10 Millionen steigen könnte, wenn wir weiter untätig zusehen, wird klar, warum dieses Thema ein Schwerpunkt des Vorschlags ist. Zwischen 2016 und 2020 stieg die Zahl der Infektionen und Todesfälle aufgrund von AMR in der EU/im EWR erheblich an. Auf ihr Konto gehen EU-weit Jahr für Jahr 35 000 Todesfälle. Hinzu kommen hohe Kosten, allein 1,5 Mrd. EUR jährlich für unsere Gesundheitssysteme.
Die EU-Kommission legte eine Empfehlung des Rates zum Ausbau der EU-Maßnahmen zur AMR-Bekämpfung vor, um Lösungen für die Gesundheit von Mensch, Tier und Umwelt zu finden. Der Rat nahm diese Empfehlung am 13. Juni 2023 an.
Der Vorschlag empfiehlt:
1. Zulassung und Überwachung antimikrobieller Wirkstoffe
- Zulassung antimikrobieller Mittel mit Maßnahmen zur umsichtigen Verwendung
- Zusätzliche Überwachung und Kontrolle des Konsums antimikrobieller Mittel, konsequentere Prävention und Bekämpfung von Infektionen; stärkere Sensibilisierung der Öffentlichkeit, Aus- und Weiterbildung von Fachkräften.
2. Umsichtige Verwendung antimikrobieller Mittel
Nur jede*r Zweite in der EU weiß, dass Antibiotika gegen Viren wirkungslos sind. Die übermäßige Verwendung und der Missbrauch antimikrobieller Mittel wie Antibiotika führen zu immer mehr Antibiotikaresistenzen.
Die Kommission setzt sich für einen umsichtigeren Einsatz antimikrobieller Wirkstoffe ein, will selbst weniger Antibiotika einsetzen und empfiehlt den Mitgliedstaaten die Festsetzung entsprechender Ziele:
- 20 % weniger Antibiotika-Einsatz in der EU bis 2030
- zusätzlich Empfehlungen für Ziele auf nationaler Ebene
3. Sicherstellung der Verfügbarkeit von Antibiotika
Der umsichtige Einsatz von Antibiotika ist für die AMR-Bekämpfung von zentraler Bedeutung, wirkt sich aber auch auf Verkaufszahlen und Investitionsrenditen von Pharma-Herstellern aus.
Daher müssen wir die Entwicklung innovativer antimikrobieller Mittel fördern und dafür sorgen, dass diese Wirkstoffe zugänglich und verfügbar sind.
Die Kommission schlägt deshalb Folgendes vor:
- eine übertragbare Zusage für Datenexklusivität, die den Entwicklern neuer antimikrobieller Wirkstoffe ein zusätzliches Jahr Marktschutz bietet, was es attraktiver macht, innovative antimikrobielle Mittel ohne direkte finanzielle Zuwendungen seitens der Mitgliedstaaten zu entwickeln
- Beschaffungsverfahren für den Zugang zu antimikrobiellen Wirkstoffen, auch zu solchen in Entwicklung
4. Weltweite AMR-Bekämpfung
Antibiotikaresistenzen können nicht von einem Sektor, einem Land oder einem Kontinent allein bekämpft werden. Das bedeutet:
- AMR im Zentrum der globalen EU-Gesundheitsstrategie belassen
- auf mehr globale Zusammenarbeit drängen, beispielsweise bei der AMR-Bekämpfung im Rahmen eines WHO-Abkommens zu Pandemieprävention, -vorsorge und -reaktion

